International Year of the Periodic Table
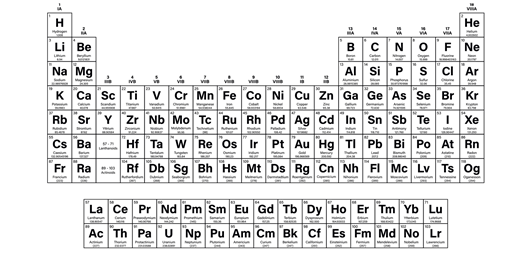
Since 1817, we’ve seen many scientific developments, including the most important one of all! The Periodic Table of the elements. 2019 was even dedicated the International Year of the Periodic Table, which marked 150 years since Dimitry Mendeleev made the discovery.
It’s a unique tool, enabling scientists to predict the appearance and properties of matter on the Earth and in the rest of the Universe. Not just a truly inspirational scientific discovery but one that transformed the way in which we view practical science in our schools and colleges. What makes Mendeleev’s discovery all the more remarkable, is that he foresaw the future discovery of other elements and set the table out in a format that would allow future scientists to enhance his work.
What is the International Year of the Periodic Table?
2019 was the UNESCO International Year of the Periodic Table, marking 150 years of the Mendeleev periodic table, something everyone who studies and works in science uses through out each stage of their learning and career.
Why is the Periodic Table so Important?
The key thing that the periodic table allows us to do is find out the properties of each element at a quick glance as it organises each one according to similar characteristics. So, instead of having to remember the properties of each element, a quick look at the table will tell us all about the reactivity of an element, whether it is likely to conduct electricity, whether it is hard or soft, and many other characteristics.
Before the discovery of all naturally occurring elements, the periodic table was used to predict the chemical and physical properties of elements in the gaps Mendeleev left on the table and since then 38 new chemical elements have been discovered.
The Periodic Table Explained
The periodic table can be a little overwhelming so, here’s a quick guide on how to read it.
- There are 18 columns, also known as groups
- There are 7 periods, these are the horizontal rows within the table
- Group 1 is the Alkali Metals (excluding Hydrogen)
- Group 2 shows the Alkali Earth Metals
- Groups 3 – 12 are the Transition Metals (excluding elements on separate rows)
- Group 17 are non-metals called Halogens
- Group 18 are the Noble Gasses
- Elements 57 – 71 are Lanthanoids
- Elements 89 – 103 are Actinoids
- Elements 93 onwards are the Transuranium elements
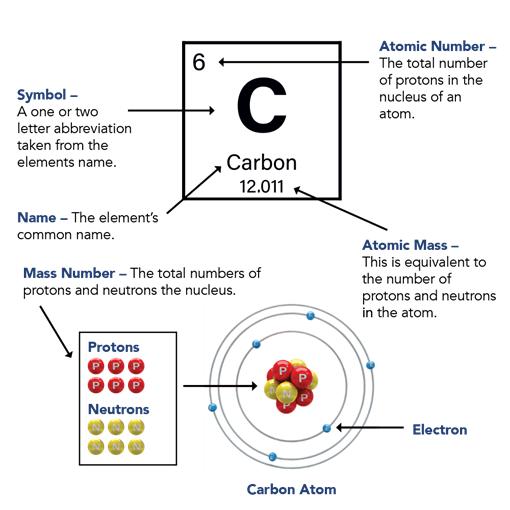
Periodic table activities and lesson ideas
Teaching the periodic table doesn’t have to be daunting, take a look at these ideas for fun and simple classroom-based activities.
- Periodic table bingo – create enough cards for your class, each one showing different combinations of elements. Pick individual elements out of a bag and the first to get a full house wins.
- Classroom display – simply create your own periodic table using folded paper so each one opens like a book and on the inside write the properties of each one. Why not divide the class into small teams so each can focus on a different group or element type? They can then report their findings back to the rest of the class, a great way for students to learn about the individual elements.
- Match everyday items to the element - for example, match salt to sodium or lithium to batteries.
- Element top trumps – cut out each of the elements, giving 10 to each student. They then battle it out using atomic numbers and mass. The person with the most elements at the end wins.
- Something’s missing – there are a lot of variations of this activity. You could ask your class to fill in the missing element symbol, a missing atomic mass or even the group name.








